When the lithium-ion battery is in a low temperature state, its available capacity is reduced and the charge and discharge power is limited. If the power is not limited, it will cause the evolution of lithium ions inside the battery, which will lead to irreversible attenuation of the battery capacity, and will bury a safety hazard for the use of the battery. The lower the ambient temperature, the lower the activity of the active substance in the battery, the higher the internal resistance and viscosity of the electrolyte, the more difficult the ion diffusion, and the slower the diffusion of lithium ions in the electrode at low temperature, which is difficult to embed and easy to come out, so that the capacity rapidly decreases, therefore, the use of low temperature will have a great impact on the battery life.
I believe we all have similar feelings, lithium batteries in winter use time is shorter than summer. It can be seen that the performance of lithium batteries is affected by the ambient temperature. Among all environmental factors, temperature has the greatest impact on the charge and discharge performance of lithium batteries. Generally, people in the lithium battery industry know that the charge and discharge state of lithium batteries is stable, and the change in temperature plays a great influence, and the charge and discharge of lithium batteries in high and low temperature environments, and the capacity retention rate of lithium batteries has declined.
It needs to be explained to you that the capacity of lithium-ion batteries at low temperatures does not disappear, but just cannot be released in the normal voltage range (≥3.0V), if the discharge cut-off voltage can continue to be extended, then the remaining capacity can be released.
The electrochemical reaction at the electrode/electrolyte interface is related to the ambient temperature, and the electrode/electrolyte interface is regarded as the heart of the battery. If the temperature drops, the reaction rate of the electrode also drops, assuming that the battery voltage remains constant and the discharge current is reduced, the power output of the battery will also drop.
Figure 1 is a schematic diagram of the discharge capacity curve of a lithium-ion battery at different low temperatures (here it is used to represent the general trend). Compared with room temperature 20℃, the capacity attenuation at low temperature -20℃ has been more obvious, to -30℃ is more capacity loss, and the capacity at -40℃ is not even half.
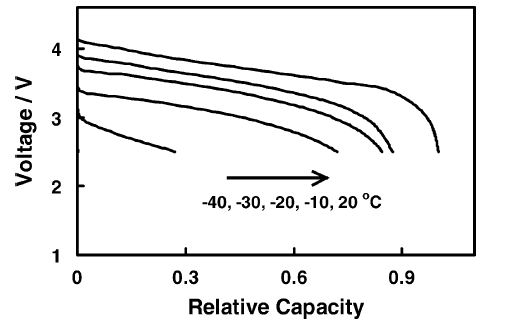
Figure 1 Capacity attenuation of lithium-ion batteries at low temperatures
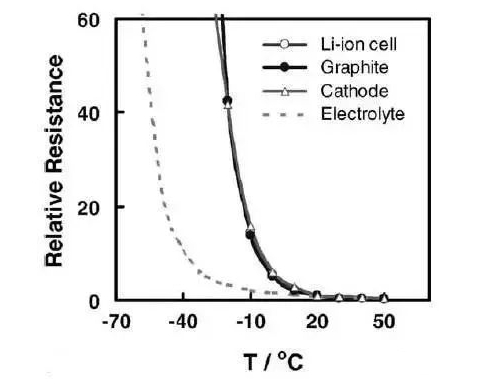
Here is a look at the factors that affect low temperature performance. By comparing the relationship between capacity and electrolyte conductivity (Figure 2), it can be seen that the lower the temperature, the lower the conductivity of the battery electrolyte. When the conductivity decreases, the ability of the solution to conduct active ions decreases, which is reflected in the increase of the resistance of the internal reaction of the battery (this resistance is expressed in the electrochemical impedance), resulting in a decrease in discharge capacity, that is, a decrease in capacity. Further, by measuring the impedance of each part of the battery (positive, negative, electrolyte), you can see the influence of each part on the battery impedance (Figure 3). When the temperature is <-10 ° C or so, the interface impedance of the positive electrode and the negative electrode (graphite as an example in the figure) increases rapidly, while the impedance of the electrolyte rises rapidly after about -20 ° C, and the combined results of these impedances show that the battery impedance rises rapidly at <-10 ° C or so (represented by Li-ion cell in the figure).
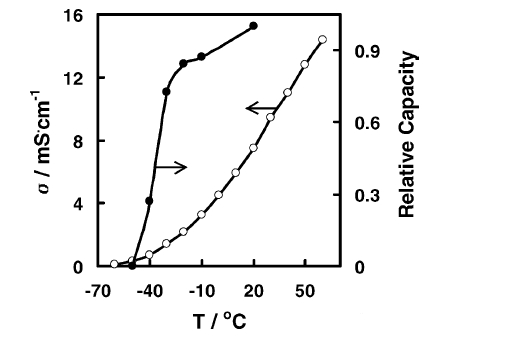
FIG. 2 Relationship between battery capacity and electrolyte conductivity at different temperatures
Figure 3 Impedance of the internal parts of the battery at different temperatures
Compared with low-temperature discharge, lithium-ion battery low-temperature charging performance is more unsatisfactory, low-temperature charging below 0 will increase the internal pressure of the battery and may open the safety valve, first of all, low-temperature charging will quickly reach the constant voltage stage, and will reduce the charging capacity to a certain extent, while increasing the charging time, not only that, lithium-ion battery in low-temperature charging, Lithium ions may not be able to be embedded in the graphite negative electrode, so as to precipitate on the negative surface to form metal lithium dendrites, this reaction will consume the battery can be repeatedly charged and discharged lithium ions, and greatly reduce the battery capacity, precipitated lithium metal dendrites may also puncture the diaphragm, thus affecting the safety performance.
The low-temperature discharge capacity of lithium-ion batteries will be reduced, but it can be restored after normal temperature charge and discharge, which is a reversible capacity loss. However, low temperature charging will cause lithium analysis, which is a permanent capacity loss. Due to the greater harm of low-temperature charging lithium, low-temperature charging of lithium-ion batteries is more strictly controlled than low-temperature discharge.
When charging in winter, the outdoor temperature is low, when the environment is below 0 ° C, the battery charging speed decreases, and even may not be able to charge, which is a normal phenomenon, please charge the battery at the appropriate ambient temperature to ensure the charging effect.